For those who are immunocompromised, the use of the affected products could potentially result in severe or life-threatening conditions such as fungemia — fungi or yeasts in the blood — or disseminated fungal infection.
The majority of consumers are not likely to experience life-threatening infections but it cannot be ruled out, according to the FDA.
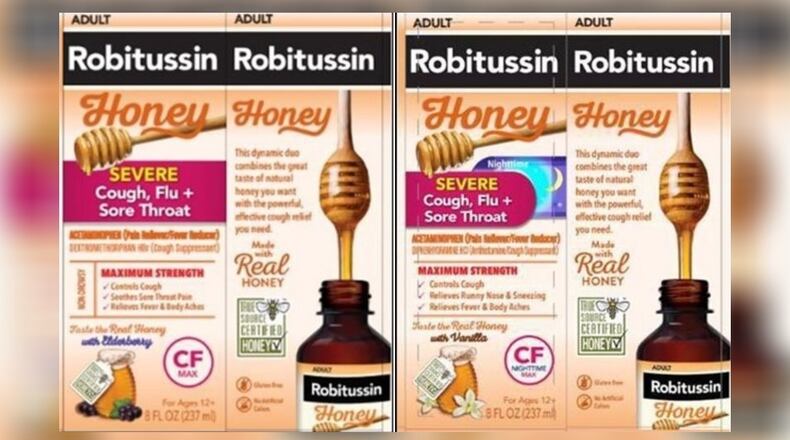
The following cough syrups are under recall:
- Robitussin Honey CF Max Day Adult, 4 ounces with lot number T10810 and expiration date Oct. 31, 2025;
- Robitussin Honey CF Max Day Adult, 8 ounces with lot numbers T08730, T08731, T08732 and T08733 with expiration date May 31, 2025, and lot number T10808 with expiration date Sept. 30, 2025;
- Robitussin Honey CF Max Nighttime Adult, 8 ounce with lot numbers T08740 and T08742 with expiration date June 30, 2026.
Haleon is notifying its distributors directly to return the recalled products. Those who have purchased the products under recall should return them to the place of purchase.
Anyone who has experienced any problems that may be related to using the product should contact a health care provider. Adverse reactions can be reported to the FDA’s MedWatch Adverse Event Reporting program online, by mail or by fax:
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
About the Author