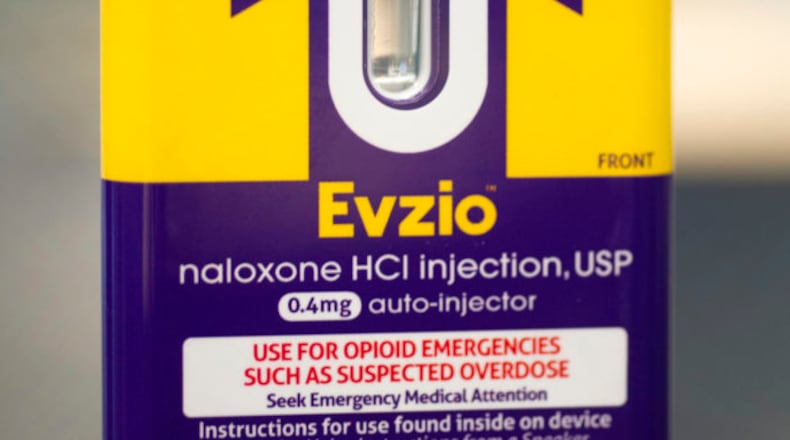
On Wednesday, weeks after news media including 60 Minutes reported on the findings of the senators’ report, kaléo announced it was introducing a generic version of its naloxone auto-injector that would cost $178 per carton, with each carton containing two auto-injectors. The product delivers naloxone in order to revive those who have overdosed on opioids and, according to kaléo, is the only FDA-approved naloxone auto-injector with voice and visual guidance that talks the user step by step through the administration of naloxone to reverse the effects of an opioid overdose.
In a press release, the company said it would be releasing the generic version of the drug in midyear 2019.
The Path Forward: Addiction in Dayton
- » Vets twice as likely to fatally OD -- what the Dayton VA is doing about it
- » ‘Life Changing Food’: This eatery hires only people recovering from addiction
- » Local chef in recovery serves up message of hope
- » New challenge for recovering addicts: Finding a job
- » Can Dayton go from 'overdose capital' to a model for recovery?
- » 'A whole new life:’ Local people share their path out of the depths of addiction, and into long-term recovery
- » Mother of 7 rebuilding family after addiction
- » A day with Dayton's overdose response team
"With approximately 130 people dying daily from opioid overdoses, we recognize that more needs to be done to improve access for patients," said Spencer Williamson, President and CEO of kaléo. "We have been working for some time with the major pharmacy benefit managers and insurers to identify solutions for removing barriers and restrictions that may impede access.”
Williamson said the company was confident that introducing the generic version of the drug “is the most efficient way to provide greater coverage of a lower-priced option – with the least amount of disruption to the healthcare system.”
The company said that EVZIO is available for $0 out-of-pocket to eligible patients with commercial insurance. However, the generic $178 version of the drug will offer a more affordable and cost-effective solution for Medicare Part D patients and plans.
Finally, the company also said it would be introducing public access pricing of the drug for $178 per carton. That pricing would be available to government agencies, first responders, health departments and other qualifying groups who treat those overdosing on opioids.
In a release highlighting the announcement, Portman called it “a positive step forward.”
“I’m hopeful that it will increase access to naloxone, a critically–important overdose reversal drug that has saved tens of thousands of lives,” he said. He and Carper are the chair and ranking member of the Senate Permanent Subcommittee on Investigations.
The subcommittee found in their November 2018 report that the company had launched a new distribution model that hoped to “capitalize on the opportunity” of “opioid overdose at epidemic levels.”
Company salesmen and women focused on ensuring doctors’ offices signed necessary paperwork indicating that Evzio was medically necessary which meant the drug would be covered by government programs like Medicare and Medicaid.
That plan worked. Taxpayers footed more than $142 million for the drug in just the last four years, the report found, despite the fact that less costly versions of naloxone exist.
The synthetic opioid drug fentanyl killed 3,431 people in Ohio last year.
About the Author